Developed in the Roots Lab at Penn State University, laser ablation tomography is a novel method that allows for rapid, three-dimensional quantitative and qualitative analysis of plant anatomy.
Strock CF, Schneider HM, Galindo-Castaneda T, Hall BT, Van Gansbeke B, Mather DE, Roth MG, Chilvers MI, Guo X, Brown KB, Lynch JP (2019) Laser ablation tomography for visualization of root colonization by edaphic organisms. Journal of Experimental Botany, 70: 5327-5342 https://doi.org/10.1093/jxb/erz271
Overview
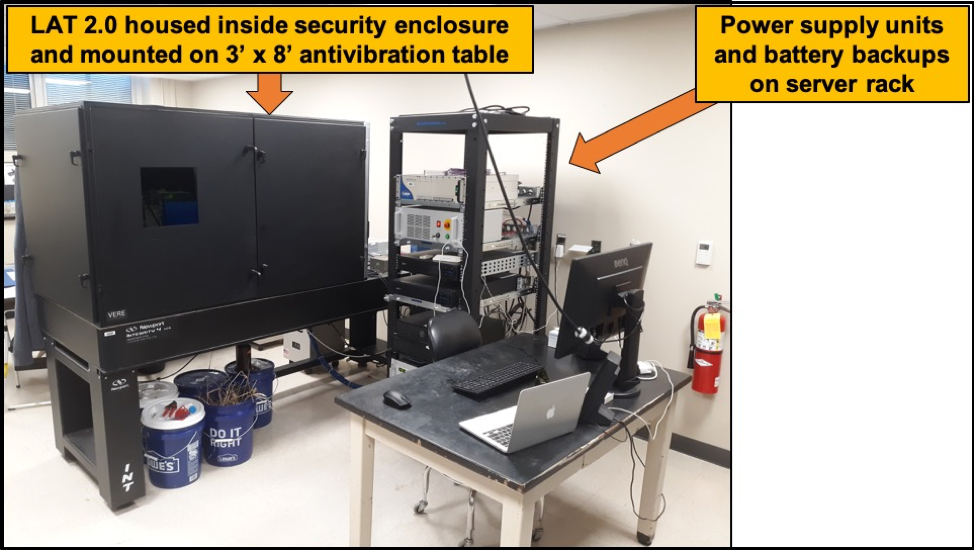
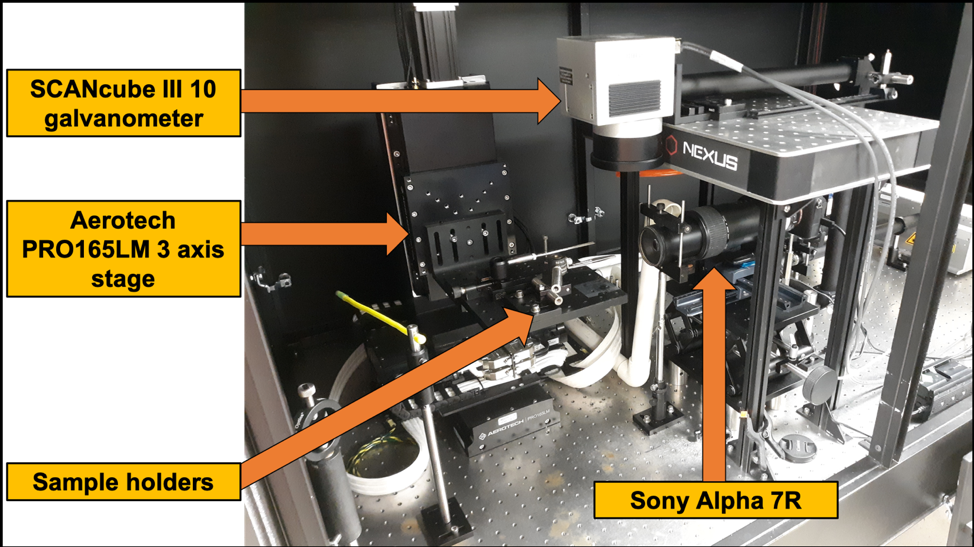
Laser Ablation Tomography 2.0 (LAT 2.0) was officially launched November 2019. The system includes EdgeWave PX-series 355nm laser with a typical pulse energy and duration of 1mJ and 10 ps, respectively. The ultrafast pulse duration of 10 ps allows our samples experience a phenomenon commonly called: athermal ablation. A level of Athermal ablation is achieved if all hardware configurations are optimally set. Coupled with this laser is a PRO165LM 3-axis stage, SCANCube III 10 galvanometer with 103mm and 160mm scanner objectives and Sony Alpha 7R camera with a 42 MP resolution. These basic components are responsible for generating the images and videos used for anatomical phenotyping and 3d reconstructions.
This system build allows users to integrate other hardware components into the system with ease as there is sufficient space for altering the layout of components. Of those components include the future integration of a hyperspectral camera which is mentioned later in another section. The simplistic design of the hardware and accompanying software amplifies users experience [compared to LAT 1.0] and offers a high degree of flexibility with regards to automation and higher throughput goals. Steps have already been made to automate the camera and stage controls through various scripts. The end goal is to integrate all the components together in a seamless platform with minimal user interaction during ablation. LAT 2.0 is equipped with different sample holder options allowing users to secure various materials for ablation. Our sample holders can hold roots, woody and non-woody stems, seeds, etc.
Automation
Our stage table is equipped with a standard optical breadboard that permits easy attachment of different sample holders, one of which is our 10-sample cassette as shown in the figure below. Samples are loaded into the stainless-steel wells to minimize pre-ablation desiccation. Importance of minimizing desiccation is directly related to overall image quality and trait extraction via RootScan. Our wells accomplish this by reducing the surface area evaporation which yields a fresh root for ablation.

Root cassette made from 3D printed PLA and stainless-steel capillary tubes.
Rear view perspective showing stage movement to push samples out of wells using pushpin design.
Side-by-side video of loaded sample cassette (left panel) and resulting ablation (right panel)
Hyperspectral Imaging
One of our research goals involves using a hyperspectral camera to distinguish cell wall components such as lignin, cellulose, pectin, xylan, suberin, etc. Our intention is to identify and quantify these components for the development of new phenological root traits for the utilization by breeders and modelers. Currently, the process is underway to demo a few hyperspectral cameras to determine their efficacy to our end goals.
Example applications
Ablation of aloe root under drought
Intra-node of wheat stalk
Fern spores on underside of fern leaf
PDF document, 1.0 MB

